In this section: Table size | Automatic renumbering and positioning of tables in the review | Types of tables | Formatting: Cell alignment | Width and height | Title | Column headings | Row headings | Table body | Footnotes: examples; positioning; multiple footnotes
Table size
For the content to display correctly when a review is published in the Cochrane Library, a table should have a maximum of 12 columns.
Tables with up to seven columns will display in the published PDF in portrait orientation. Tables with 8 to 12 columns will automatically be displayed in the published PDF in landscape orientation.
RevMan will not allow you to exceed the maximum size of 25 columns.
Automatic renumbering and positioning of tables in the review
In the read-only views generated using the options 'View' [a version] and 'Submission preview' (focused review format only), and in the PDF article, tables are renumbered to reflect the position in which they are linked in the review.
In the published review PDF, tables are placed at the end of the paragraph in which the link appears. If a table is linked more than once in the text, the second and subsequent links will point to the table at its first appearance in the text. Readers need to use the browser's back button to return to the section they were navigating before being redirected.
The positioning of tables cannot be previewed in RevMan.
Types of tables in RevMan
Tables in the main text of the review | |||
|---|---|---|---|
Table type | Description | Where to insert/edit in RevMan | Permitted formatting |
Overview of included studies and syntheses (OISS table) | Give brief details of key study characteristics that aid readers' understanding of the results of the review, including which studies provided results for which outcomes and meta-analyses. Where it makes sense to do so, group studies according to particular characteristics, outcomes or interventions. You can add more than one table if necessary to avoid over-long tables. | In Default view, create an Additional table, and give it the title, 'Overview of included studies and syntheses'. | Same as available within main text plus heading cells, cell alignment and cell merge. Footnotes permitted |
Additional tables | Used for presenting limited amounts of information in tabular format within the main body of the text. | In Default view, create an Additional table; name and fill as appropriate. | Same as available within main text plus heading cells, cell alignment, and cell merge. Footnotes permitted |
Summary of findings tables | Present summary of the results for the 7 most important outcomes in the review. When the review is published, these tables follow on after the Abstract, Plain language summary and Authors' conclusions. | Generate and edit in GRADEpro GDT (the default setting in focused review format). Summary of findings tables will be synced with RevMan. Edit in GRADEpro GDT only. Generate table in GRADEpro GDT and copy and paste into RevMan table. In Default view, cliick on 'Summary of findings' in the navigation pane > 'Add table' > 'Using RevMan Web' and copy table from GRADEpro GDT. Edit in RevMan. Generate and edit table in RevMan. In Default view, cliick on 'Summary of findings' in the navigation pane > 'Add table' > 'Using RevMan Web' | Limited editing functions in GRADEpro GDT. (See Knowledge Base). If copied from GRADEpro GDT or generated within RevMan, same as available within main text plus heading cells, cell alignment and cell merge. Footnotes permitted |
Tables in Supplementary materials | |||
|---|---|---|---|
Table type | Description | Where to insert/edit in RevMan | Permitted formatting |
Characteristics of studies tables (included, excluded, ongoing, awaiting assessment) | Present the characteristics of the different types of studies. Tables for included and ongoing studies, and studies awaiting assessment, have a number of predefined attributes (methods, participants, interventions, outcomes, notes). Excluded studies tables give brief reason(s) underlying decisions to exclude studies from the review. | In Default view, select 'Studies' and desired study type. Select 'Add study' to insert a study, and 'Edit study' to edit the text. It's possible to move between tabs that document different types of information about each study using the 'Next' and 'Previous' buttons. | Same as available in main text Footnotes permitted |
Risk of bias tables | Used to present risk of bias judgements and support for judgement for each study. RoB 1 (Cochrane's original risk of bias tool) is part of the Characteristics of included studies supplementary material. RoB 2 is presented in the Risk of bias supplementary material. (These are called 'Assessment of methodological quality tables' in diagnostic reviews.) | RoB 1: in Default view select Studies > Included > Edit study, then select Risk of bias from the choices at the top of the page. RoB 2: see Cochrane Methods | Same as available within main text Footnotes permitted |
Additional tables | Only the most important tables (i.e. those that present information about the review's findings) should remain as 'Additional tables'. Other tables should be moved to the Supplementary materials in focused-format reviews, or Appendices in long-format reviews, and referenced with a hyperlink. For example, risk of bias for non-randomized studies of interventions, or a glossary of technical terms. | Generate and edit table in RevMan. In Default view, click on 'Tables' in the navigation pane > 'Add table', then select the number of rows and columns. Type into the cells or copy and paste from outside RevMan. | Same as available in main text plus heading cells, cell alignment and cell merge. Footnotes permitted |
Formatting options
In addition to the formatting that is available within all of the main text, some tables have three further options:
- heading cells, which applies heading style to the cell (shown as bold in RevMan);
- cell alignment, which allows a choice of horizontal and vertical alignments; and
- cell merge, which allows adjacent cells to be merged together to create a single cell.
Formatting should be consistent within a single table. As with other parts of a Cochrane review, the visual presentation of tables will change during the publication process; for example, it is important not to use the ‘Enter key’ within a single block of text (e.g. in a single word if the word runs over two lines) because the text layout will also change during the publication process.
Cell alignment examples
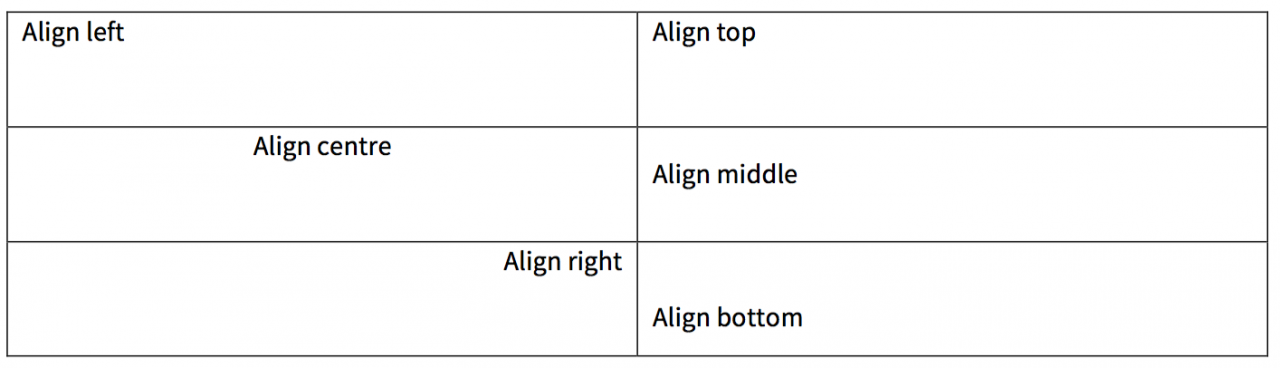
Width and height
The width and height of tables or individual cells cannot be specified. Instead, cells (and thereby row, columns, and tables) expand automatically to fit the content.
Table title
The title should be concise and reflect the table content. Use sentence case without a full stop at the end.
Column headings
Column headings should be in sentence case and formatted using ‘Toggle heading/cell’, which applies heading formatting to the cell. This also applies if there are nested column heads (i.e. two rows of column headings); the top heading in nested column heads is likely to be in a series of merged cells that span the relevant columns.
Horizontal alignment: in general, the heading cells should be left-aligned. If the table contains nested column heads (see above), then the top row should be centre-aligned.
Vertical alignment: column headings should be bottom-aligned (instead of top- or middle-aligned).
Row headings
Tables may or may not have headings for each row. If so, these should be in sentence case and formatted using 'Toggle heading/cell', which applies heading formatting to the cell.
Horizontal alignment: the row headings should be left-aligned unless there is good reason to do otherwise.
Vertical alignment: in general, row headings should be top-aligned.
Table body
Use sentence case if the content is all or mainly text. Use numbers instead of words in tables (see Exceptions to basic rules for numbers and ordered events less than 10). Only use full stops to end blocks of text when the block ends with a full sentence. It is customary to use a dash (en-dash) when writing ranges in tables but it is acceptable to write 'to' as long as the style is consistent within and across tables. For example, 10 mg-20 mg or 10 mg to 20 mg.
Horizontal alignment: in general, the body cells should be left-aligned.
Vertical alignment: vertical alignment of the body cells should be top (instead of middle or bottom). There may be occasions when the cells should be bottom-aligned, but this should only be done when it makes sense visually.
Blank cells: avoid blank cells in a table. Insert an em-dash or ellipsis if the column heading does not apply to the cell, or use NA (not applicable) or ND (no data available) if a distinction is needed. Remember to explain these two abbreviations in the footnotes.
Footnotes
Footnotes are a convenient way to define abbreviations and acronyms or display other explanatory notes (see Examples of table footnotes below). Use superscript lower-case letters to denote footnotes. Where a footnote symbol follows punctuation, place the footnote symbol immediately after the punctuation mark unless it is a dash or closing bracket. Where a footnote refers to a specific point within a sentence, place the footnote symbol immediately after the relevant phrase (see Examples of correct and incorrect positioning of footnote symbols below). The placement of the footnote symbol should go from left to right, followed by top to bottom. When a footnote refers to the whole table, for example referencing the source of the table content, the footnote symbol should go at the end of the table title.
Each footnote needs to be explained. Repeat the superscript letter immediately under the table and follow it with the explanatory text. There is no space between the superscript letter and the explanatory text. Start each footnote on a new line, using a soft return between footnotes (i.e. hold the shift key when pressing return). Footnotes may or may not be full sentences, but if they are full sentences they should end with a full stop.
Examples of table footnotes
Use a superscript letter for a footnote.a | The order of footnotes should go left to right, followed by top to bottom.b |
A third footnote could be used here.c | — |
Examples of correct and incorrect positioning of footnote symbols
Correct | Incorrect |
The analysis does not include the full study.a The doses were inconsistent (from 0.5 mg to 10 mgb) and reported only once a day. The dosesc were inconsistent (from 0.5 mg to 10 mg). | The analysis does not include the full study a. The doses were inconsistent (from 0.5 mg to 10 mg)b and reported only once a day. The doses were inconsistent (from 0.5 mg to 10 mg)c. |
How to format multiple footnotes
If you need to add multiple footnotes to one statement, use commas to separate the footnote letters but do not add spaces after the commas.
Here is an example.a,b,c
In cases where there are a large number of footnotes to be applied, it is acceptable to display a range with a hyphen. This will be a judgement based on the context in the table and should be applied consistently within the table.
Here is another example.b-h
If there are a large number of footnotes but the range is not continuous, then a mix of commas and hyphens is necessary.
Sometimes you may need to take this approach.b,c,f-j
