GES Cochrane Consumer Stipends





Rebecca Costantini, PhD (UX consultant, Wiley), Rachel Craven (Product Lead, Cochrane Library), Samantha Cox (Information Specialist, Cochrane Library), and Colleen Finley (Technical Product Manager, Wiley) discuss the results from recent interviews with information specialists and librarians about their search strategy process.
Search strategies are used to locate, retrieve, and organize literature across databases. Librarians and information specialists often create complex search strategies to support multi-line searches for medical staff and researchers, standardize search strategy approaches for larger teams, and evaluate medical evidence (among many other things!). These search strategies often involve specific concepts, syntax, filters, subject headings, or limits that drive the search. The Cochrane Library has dedicated tools to facilitate quality search strategies, including advanced search, which contains search, search manager, medical terms (MeSH), and PICO (Population, Intervention, Comparison, Outcome) search.
We are always looking to improve our tools and services to help users unlock the power of Cochrane evidence, so in November 2023, we interviewed 12 information specialists and librarians from around the world to learn more about their search processes and what they need from us.
What did we learn?
The search process
Many of the information specialists and librarians we spoke with begin their search strategies with a few tasks, including:
Search translation tools are sometimes used to translate initial search strategies so that they are compatible with other databases. They assist in translating search strategies across multiple databases, especially for larger searches.
Internal processes are often established to organize and standardize how search strategies are documented. Templates, folder structures, and documents are shared among colleagues and teams; and are sometimes mandated by the organization they work for and the agencies they serve.
Choosing a search tool
Information specialists and librarians will first consider database scope, and often prioritize a flexible user interface when choosing search tools. The syntax, and available operators, fields and navigation will be an important consideration. For basic scoping, simpler approaches are favored. For searches for systematic reviews, for example, Cochrane Library’s search manager is the tool of choice. Additionally, the content of search strategies affects the choice in search tool, especially for semantically complex searches.
An efficient search process
The information specialists and librarians we spoke to often seek accessible, time-saving search features to facilitate longer, more complex searches. Search tools need to meet the requirements of existing process and be comprehensive. Information specialists and librarians do not have the resources to try new tools if they do not support their current processes. Some of the questions that resulted from these conversations revolved around how AI could play a bigger role in the future of building search strategies.
Conclusions
Generally, we heard from users that the Cochrane Library search tools - particularly advance search and search manager - provide information specialists and librarians with all the tools they need, and almost everything they want to perform their day-to-day tasks.
However, we were interested to learn that information specialists and librarians are not always aware of these Cochrane Library search features; or are unable to utilize them fully because of restrictions imposed by internal processes and policies. We also learned that users sometimes find that our interface is not always intuitive, because they are more familiar with other tools that work differently.
Information specialists and librarians also told us that they valued trust, flexibility and functionality over performance (speed) on Cochrane Library, but that reliability is sometimes an issue; and that improvements in speed of performing tasks would be valued.
Next steps
We have been working with our publisher, Wiley, and their software development partner, Highwire, to improve performance, the speed at which complex searches run; and reliability, allowing users to run complex searches and get the results they need.
We made several changes in early 2024 that resulted in incremental but significant improvements in these two key areas, and we hope these changes will address the needs of librarians and information specialists.
Our plans for 2024 involve making our training materials more useful and accessible to help information specialists and librarians get the most out of the Cochrane Library’s search tools. We will continue to look at ways of optimizing our search tools to support librarians and information specialists in performing their day-to-day tasks as efficiently as possible.
We continue to work on evolving the Cochrane Library search tools, and really value feedback from our users.
If you are interested in participating in future feedback opportunities about your Cochrane Library experience, complete this form.

Cochrane has made significant changes and improvements to Cochrane’s review production model, which has led to the creation of our new focused review format. This isn't just a tweak - it's a complete overhaul to make life easier for our authors and improve how our systematic reviews are conducted and presented.
Take a look below to find out more, and watch our two-minute video explaining the changes with Cochrane’s Ella Flemyng.
A new era for Cochrane Reviews - dates you need to know
From 1st April 2024, all new submissions should be in this new focused review format. If you are submitting after this date, please log into RevMan now and switch to the focused format. This is ahead of our big changeover on 1st June 2024, when all systematic reviews in RevMan will automatically convert to the focused format.
For those with protocols and reviews in the editorial and production stages as of June 1st, don't worry. Your RevMan file won't switch to the focused review format until after the final editorial decision, either publication if accepted, or rejection.
The Focused Review Format: Work Smarter, Not Harder
The focused format is designed to streamline the review process and make things simpler and clearer for our Authors. Not only will it simplify reporting and speed up the development of reviews, but it will also amplify the impact of published reviews.
From June 1st, all reviews in RevMan will automatically have access to study centric data management, which brings a host of benefits to authors. Study centric data will be available in RevMan files that use the following review types: Intervention, Methodology, Flexible. You'll be able to reuse study data between analyses and import study data from Excel and Covidence. You can read more here about the difference in the data management options.
If you're eager to get ahead of the curve, you can activate study centric data on your review dashboard now, before it automatically goes live on June 1st. Find out more in the RevMan Knowledge Base.
A consistent, clearer experience for all our Authors
Cochrane is making these changes to ensure all authors enjoy a consistent, simplified process. It will also make training and support much more straightforward. Cochrane’s author guidelines provide essential guidance on preparing your manuscript for submission, including how to check if your submission meets Cochrane standards and policies. For further details, don't hesitate to consult the RevMan Knowledge Base and Cochrane's author guidelines.
Do get in touch with Cochrane’s support team with any issues or questions!

The Cochrane Support team is the first point of contact for any queries coming from the Cochrane Community, including the Central Executive Team and Cochrane group staff, authors, editors, consumers, and the general public. If you have a question about Cochrane, we have an answer!
We maintain multiple resources for the Community to help you learn about Cochrane and answer questions that might arise:
If needed, we connect with second-line support teams that work on RevMan Web, Editorial Manager, and other Cochrane systems.
In addition to providing answers related to Cochrane’s technical systems, Cochrane Support also help users with challenges logging in to their Cochrane accounts and answer queries related to governance, elections, Cochrane membership, conflict of interest policies and procedures, and more!
We welcome queries in any language, and our international team can respond to you in English, French, Spanish, and Portuguese.

Our Community says...


Don't hesitate to reach out to Cochrane Support; we are always happy to help.

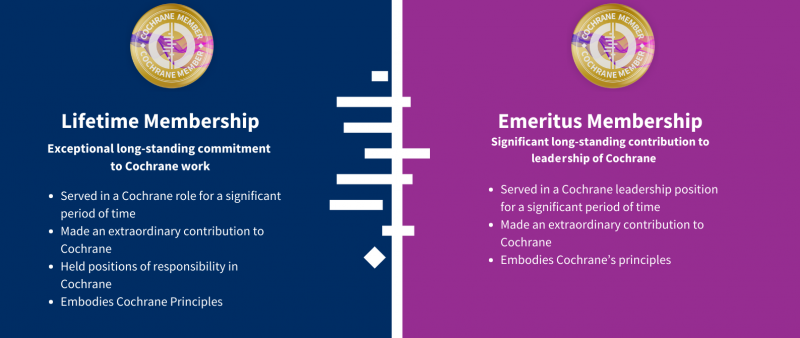
Cochrane is proud to recognize the extraordinary contributions of individuals who make an exceptional, long-standing contribution to Cochrane’s work and leadership with lifetime and emeritus membership.
Cochrane's strength is in its collaborative, global community. Our 100,000+ members and supporters from more than 130 countries work together to produce credible, accessible health information and help inform health decision making. Though we are spread out across the globe, our shared passion for health evidence unites us.
Cochrane’s Membership scheme helps reward everyone who helps provide produce and disseminate Cochrane evidence, as well as provide leadership for our strategic goals. Membership is a valuable addition to résumés, gives you voting rights, and opens opportunities for getting involved in governance and learning opportunities.
We are proud to recognise extraordinary contributions to Cochrane with Lifetime and Emeritus Memberships. These will be awarded to those who have contributed to Cochrane’s success over many years and are now ending their active time with Cochrane.
Nominate a Cochrane Colleague
Cochrane values diversity and inclusivity, and our Lifetime and Emeritus Members should reflect the rich diversity of our global community. We welcome nominations for individuals of various geographical locations and genders, and particularly endorse a balanced representation from all around the world.
If you know a Cochrane Colleague who has made an exceptional long-standing commitment to Cochrane’s work but is now ending their active time with Cochrane, we invite you to make a nomination.
Nominations will be collated and then put forward for consideration to the Membership and Awards Committee of the Governing Board.

Are you interested in joining the Governing Board?
Two elected members of the Governing Board (Juan Franco and Tamara Kredo) come to the end of their first term of appointment this year and so elections will be held. Candidates must be a Cochrane Member as of 15 November 2023 to be eligible to stand in this election, membership is defined as having accepted the Terms & Conditions of Cochrane Membership. You can check your membership status here.
The Cochrane Collaboration is a charity, registered with the Charity Commission (which regulates charities in England and Wales). Members of the Governing Board are the charity’s Trustees and Directors of the charitable company. Trustees are legally responsibility for the organization’s management and administration.
This year the Board is looking for candidates who can demonstrate skills and experience in one or more of the following areas:
The Board is committed to improving the organizations’ approach to equity, diversity and inclusion. It recognises that having members with a broad mix of skills and knowledge as well as a range of perspectives and lived experiences will help the Board to be innovative, flexible, better able to adapt to a changing environment and address future challenges.

Dr Thomas Chalmers was an outspoken advocate of randomised trials, whether at the bedside, at professional meetings, in class or in situations pertaining to his own life. His creativity spanned his entire career, influencing clinicians and methodologists alike. He is perhaps best known for the notion ‘randomise the first patient’, his belief that it is more ethical to randomise patients than to treat them in the absence of good evidence. In his later years, in arguably his most important work, Tom and his colleagues showed that had information from RCTs been systematically and cumulatively synthesised, important treatments such as thrombolytic therapy for myocardial infarction would have been recognised as useful much earlier.
The Thomas C Chalmers Award was initiated by Tom Chalmers himself and further supported with individual donations from friends and family to celebrate and recognise Tom's interests and achievements. The prize is awarded to an early career investigator presenting on methodological issues at the Cochrane Colloquium. The presentations must demonstrate originality of thought, high-quality science, relevance to the advancement of the science of systematic reviews and clarity of presentation.
The Thomas C Chalmers Award for Best Poster Presentation goes to Mayara Silveira Bianchim, Patient and Public Lead for the Centre of Population Health and Research Officer at Bangor University. May is currently based in Swansea, Wales.

May won for her poster presentation called ‘Co-producing with children and young people on a meta-ethnography on experiences of chronic pain, treatments and services'
Background: Childhood chronic pain is a worldwide public health issue. We conducted a qualitative evidence synthesis using meta-ethnography, with patient and public involvement (PPI) in all review stages. We investigated the experiences and perceptions of children with chronic pain and their families of chronic pain, pain treatments and services. PPI in every stage of a systematic review or evidence synthesis is rare. We will co-present with young people with chronic pain and parents.
Objectives: To involve children with chronic pain and their families and other key stakeholders in the meta-ethnography study design, analysis, interpretation and dissemination to ensure salience of findings. To describe involvement methods and impact.
Methods: Thirty-three stakeholders, including children, parents, health professionals and third-sector representatives, took part as co-producers via workshops, meetings, email, and conference calls. They participated in developing the grant proposal, finalising the review protocol, making decisions about sampling and organising studies for synthesis and interpreted and disseminated findings. We created innovative methods to involve children and stakeholders in analysis and interpretation, e.g., we conveyed screening results using infographics and developed cartoons to show preliminary findings to facilitate discussion during workshops to clarify, interpret and/or address gaps in data.
Results: Stakeholders suggested additional sources for the literature search strategy, decided we should include non-UK studies in the sample, and agreed that we should group studies by pain condition for synthesis. PPI provided different perspectives on and interpretations of ambiguous data, challenged the review team’s interpretations and filled gaps in data based on their experiences. A young person with chronic pain co-presented at a nursing conference, and PPI co-developed an animated cartoon to disseminate findings to children and young people.
Conclusions: We believe this is the first meta-ethnography to involve patients, parents and other stakeholders as partners in every stage from inception to dissemination. Their involvement in study design, analysis, interpretation and dissemination ensured the relevance and usefulness of the review outputs, clarified ambiguous (and controversial) findings and was key to producing meaningful findings to enhance policy and practice.
We interviewed May to gain insight into her thoughts on the significance of this prestigious recognition.
What made you decide to enter for this prestigious Cochrane award?
My research was all about finding innovative and creative ways to co-produce research with children and their families. I entered the award because I believe in the impact and benefits of co-producing research with the public.
Can you tell us a little more about what made you decide to enter for the award and the process involved?
I’m part of a team led by Dr. Emma France that co-produced a study investigating children's experiences with chronic pain. I developed innovative methods such as cartoons and infographics, to help us collaborate with children and their parents. I decided to enter the award because I firmly believe in the importance and impact of collaborating with members of the public to keep the research grounded and produce meaningful outcomes.
What does this award mean you, personally?
I’m honoured and humbled to have received the award. Cochrane is recognised as the highest standard in evidence-based health and care research. This award is very meaningful as it solidifies all the hard work from our patient and public members and the research team. I’m proud of us and excited about our future work together.
What’s been the impact to you, and your work, winning the Award?
I’m hoping to continue developing this research and translate it into practice during a Research Fellowship. The award recognises the value and significance of continuing this work and it will be key to underpinning a future fellowship application.

Dr Tom Chalmers was an outspoken advocate of randomised trials, whether at the bedside, at professional meetings, in class or in situations pertaining to his own life. His creativity spanned his entire career, influencing clinicians and methodologists alike. He is perhaps best known for the notion ‘randomise the first patient’, his belief that it is more ethical to randomise patients than to treat them in the absence of good evidence. In his later years, in arguably his most important work, Tom and his colleagues showed that had information from RCTs been systematically and cumulatively synthesised, important treatments such as thrombolytic therapy for myocardial infarction would have been recognised as useful much earlier.
The Thomas C Chalmers Award was initiated by Tom Chalmers himself and further supported with individual donations from friends and family to celebrate and recognise Tom's interests and achievements. The prize is awarded to an early career investigator presenting on methodological issues at the Cochrane Colloquium. The presentations must demonstrate originality of thought, high-quality science, relevance to the advancement of the science of systematic reviews and clarity of presentation.
The Thomas C Chalmers Award for Best Long Oral Presentation goes to Peter Godolphin, Senior research fellow and part of the meta-analysis programme at MRC Clinical Trials Unit at University College London. London, UK.

Peter won for his long oral presentation called ‘Reliably estimating interactions and subgroup effects in aggregate data meta-analysis'
Background: A key question for meta-analysis is to reliably assess whether treatment effects vary across different participant subgroups. Traditionally, these interactions have been estimated using approaches known to induce aggregation bias, so we previously recommended a within-trial approach to provide unbiased estimates for binary or ordered-categorical patient-level treatment-covariate interactions. However, patients, clinicians, and policymakers need reliable estimates of treatment effects within specific covariate subgroups, on relative and absolute scales, in order to target treatments appropriately, which estimation of an interaction effect itself does not provide. Objective and
Methods: Our objective is to describe further developments to the “within-trial” framework by providing practical methods to (1) estimate within-trial interactions across two or more subgroups; (2) estimate subgroup-specific (“floating”) treatment effects that are compatible with the within-trial interactions and make maximum use of available data; and (3) clearly present these data using novel implementation of forest plots. We describe the steps involved and show how the methods can be applied using detailed aggregate (“summary”) data either extracted from trial publications or obtained directly from trial authors. We demonstrate the within-trial framework by applying it to two examples taken from previously published meta-analyses in which detailed aggregate data were available.
Results: In our first example, a meta-analysis of the effects of interleukin-6 antagonists on outcomes for patients hospitalised with COVID-19, we show how the method can be used for a binary covariate. Our second example, a meta-analysis of the effects of postoperative radiotherapy on survival of patients with non-small cell lung cancer, allows us to demonstrate the framework for a categorical covariate with three levels. We demonstrate how to implement these methods using a newly developed command (metafloat) in Stata.
Conclusions: Our within-trial framework allows straightforward estimation of a range of within-trial treatment-covariate interactions, compatible subgroup-specific treatment effects, and corresponding forest plots to clearly and effectively demonstrate how treatment effects differ across patient subgroups. Patient, public, and/or healthcare consumer involvement: One patient and one member of the public are involved in the dissemination of the results of this study, including advising on how best to present the methodology to various audiences.

The 2023 Cochrane Colloquium's closing plenary featured the presentation of the award by Ian Saldanha. We interviewed Peter afterwards to gain insight into his thoughts on the significance of this prestigious recognition.
What made you decide to enter for this prestigious Cochrane award?
It was a no-brainer. As an early-career academic I’ve quickly learned to keep my eyes open for these sorts of opportunities, and to just keep trying. With this award being so prestigious, it was an easy choice to decide to enter!
Can you tell us a little more about what made you decide to enter for the award and the process involved?
The process itself was really simple: when I submitted my abstract there was just a box to tick, saying I was eligible. At the conference itself I just gave my presentation as normal; I wasn’t aware of being judged (any more than usual)!
What does this award mean you, personally?
It means a lot. I’m delighted that the methods that colleagues and I have developed have been well received by such an important organisation, and I’m glad that I explained them well enough for people to understand! We think that we have a neat solution to an important problem: in a nutshell, we’re trying to ensure that subgroup analysis doesn’t suffer from something called aggregation bias, and it’s great to see that others are interested.
What’s been the impact to you, and your work, winning the Award?
It’s early days but already I’ve been contacted by numerous people asking to share my slides or provide more details on our method. This is exactly the reason why we try to develop new practical methodology - so that the evidence synthesis community can use it! The recognition from Cochrane I hope will just further help to disseminate this method to others and help to generate better evidence on subgroup analysis for Cochrane reviews.
What would be your message to other colleagues, Cochrane collaborators, who are considering entering for this particular award this year? Any advice for their entry?
Definitely enter, you’ve got nothing to lose and the process is simple! Some advice that was given to me, that I’d like to pass on, is that if you’re doing a statistical talk (like mine), try to limit the amount of formulas on the slides. And where you do have them, make sure you explain what everything means. It’s easy to assume that everyone can follow the maths, but in reality, keeping things simple often seems to work best. Very best of luck for everyone entering next year!

Anne Anderson was a contributor to the stream of thinking and effort that gave birth to evidence-based health care. A clinically qualified reproductive physiologist, Anne had an active interest in women’s health, co-editing the first edition of Women’s Problems in General Practice with Ann McPherson and contributed to Effectiveness and Satisfaction in Antenatal Care (1982), edited by Murray Enkin and Iain Chalmers. She was discussing with Marc Keirse and Iain Chalmers the possibility of co-editing a companion volume on elective birth, however her premature death from breast cancer in 1983 ended her involvement. Iain Chalmers, Murray Enkin and Marc Keirse went on to publish Effective Care in Pregnancy and Childbirth (ECPC) in 1989, dedicating the book in part to Anne. ECPC, through its systematic approach to assessing the research literature, is widely acknowledged to have led to the development of Cochrane.
The goal of the Anne Anderson Award is to recognize and stimulate individuals contributing to the enhancement of women’s visibility and participation in Cochrane’s leadership. In the footsteps of Anne Anderson, many outstanding women continue to contribute and inspire other women to improve health knowledge for the good of their communities. The Award recipient designates the cash award to assist another woman from a low-resource setting with her own Cochrane activities.
The 2023 Anne Anderson Award goes to Madelon van Wely.

Dr van Wely has been involved in Cochrane for more than 25 years as an author, reviewer, editor and educator. She is passionate in motivating early career clinicians and researchers, especially women, to be involved in Cochrane’s evidence-based research to improve women’s health. She has directly mentored one female managing editor who is responsible for the production of ~25 Cochrane reviews per year across the Gynaecology & Fertility Group and the Sexually Transmitted Infections Group, and has directly mentored junior clinicians or researchers as authors in 28 Cochrane reviews (including >60% female mentees).
Dr van Wely oversees two Cochrane groups in the area of women’s health (Gynaecology & Fertility Group & Cochrane Sexually Transmitted Infections Group). She promotes diversity and inclusiveness, and advocates for the enhancement of women’s visibility within Cochrane. She is currently supporting 25 female internationally recognised leading experts in women’s and reproductive health across >15 countries serving as Cochrane editors, comprising of >60% of the editorial board members across the two Cochrane groups.
Within Cochrane, Dr van Wely has been working as the co-ordinating editor of the Cochrane Gynecology and Fertility Group Satellite in Amsterdam since 2018 and also the co-ordinating editor of the Cochrane Sexually Transmitted Infections Group since 2023. Outside Cochrane, she has been working as a principle investigator at Amsterdam UMC (formally AMC Amsterdam) and has supervised 33 PhD and masters students (31 females) and 28 undergraduate students (26 females) in women’s and reproductive health research. She has been working as a senior methodologist at Women’s Clinic in Amsterdam and the Dutch Society for Obstetrics and Gynaecology Consortium since 2005, and has been holding senior editorial roles leading multiple top journals in the area of women’s health since 2012 (Human Reproduction Update, Human reproduction and Fertility & Sterility).
At the beginning of the Covid pandemic, Dr van Wely initiated a database on maternal and neonatal outcomes in CoVID-19 infected women, resulting in high daily usage. This database was subsequently incorporated in a living systematic review resulting from the close international collaboration between multiple Cochrane units (Cochrane Gynaecology and Fertility Netherlands Satellite and Cochrane Madrid) and WHO Collaborating Centre for Global Women’s Health (Allotey et al BMJ). Dr van Wely is leading a program to translate and disseminate evidence from reviews published by the Gynaecology & Fertility Group and Cochrane Sexually Transmitted Infections Group as user-friendly summaries and infographics for consumers. Dr van Wely was also involved in the development of the core outcome set and research priorities for future infertility research, as well as the screening methods to detect data integrity and fraud to promote better trusted evidence in Cochrane.

The 2023 Cochrane Colloquium featured the presentation of the award by past winner Tiffany Duque. We asked Madelon afterwards about her thoughts on the significance of this prestigious recognition.
Madelon said, "I am truly honoured to have received the Anne Anderson Award! This recognition motivates me to continue my work at Cochrane and focus even more on providing opportunities for female researchers. Thank you for this wonderful recognition."

Dr. Chris Silagy was the founding Director of the Australasian Cochrane Centre and instrumental in Cochrane's development and success. Chris was energetic, positive and inspiring. Before his death in December 2001, Chris expressed a wish for a Fund to be established, to be held by the Monash Foundation. Chris initiated this fund with his own contribution, and requested donations be made to it instead of flowers or other tributes after his death.
Chris requested that this Fund be used to recognise contributions to Cochrane in ways that are often insufficiently recognised; for example, providing administration, management, Colloquium organisation, communication and motivation - in short, the 'glue' that helps to keep Cochrane together.
The Chris Silagy Prize is given to an individual who has made an extraordinary contribution to Cochrane, made a contribution that exceeds the expectations of their employment, made a contribution to Cochrane that would not be recognised outside the scope of this Prize, and has been identified by their peers as consistently contributing to a spirit of collaboration.
The 2023 Chris Silagy Prize winner is Muriah Umoquit, Senior Communications Officer at Cochrane.

This prize was presented by past winner, Anna Noel Storr. Anna said, "This prize is awarded at each Colloquium to a person who has made an extraordinary contribution to Cochrane's work and also someone who may not otherwise be recognized - the 'glue that helps keep Cochrane together'. This year's winner fits the definition perfectly. Muriah has worked well beyond the confines of her job description. She doesn't just stick to what we have done before but has looked for ways to be more creative in communication; to innovate and support other people's innovation. She achieves an enormous amount for one person and however busy she is, she makes time to not only keep up with the volume and quality of her own output but also supports others in their own communications work. It's a great pleasure to congratulate this year's winner, Muriah Umoquit'.

Muriah shared her thoughts on winning the award: "Thank you. And a special thank you to Chris’s family and supporters. What an extraordinary honour and I am certainly among good company given the past winners of this Prize.
At the #CochraneLondon Colloquium, I had a nice chat with Jini Hetherington, the very first recipient of the Chris Silagy Prize. She asked me how I came to Cochrane and I told her that I came as a 'fan girl'. However, it was not as scandalous as Jini initially thought and this was a very important reminder to always use plain language! I came to Cochrane as a researcher in love with the methodology and high standards. And as a consumer grateful for the summaries of evidence to help guide health decisions. Over the past 8 years, I got to see the inner workings of Cochrane and the many projects and activities. The collaborative nature, dedication, and passion of the Community has always been something that has inspired me.
As a communication professional, it's been a delight working with my past team members to help upscale the knowledge translation capacity within Cochrane. I've enjoyed helping the Community share their journey through #MyCochraneStory and have been excited with them as we disseminated countless achievements and milestones in news stories, press releases, and social media posts. I've also had the opportunity to work on many side projects - infographics for COVID evidence, the Cochrane Book Club, the creation of several external science communication courses, and seeing the #BetterPoster templates rolled out at this year's Colloquium.
Chris had always said Cochrane’s success was largely due to the Community’s selflessness and collaborative outlook, often by those working in under-recognized roles. I’d like to use this platform to personally say stop. Continue that good stuff of course but stop being unrecognized. Stop being modest. Share your successes, your passion, and your stories. It’s your stories of how you got involved and what you’re doing now that are going to inspire others. And I’m here eager to help you tell these stories and celebrate with you. Together, let's continue to write the unfolding chapters of Cochrane."